Tens of thousands of new patients are affected with liver cancer in India yearly with the prospect of a very limited lifespan. Dr. Jaya Shukla has developed an injectable (transarterial) radioactive therapy to downgrade or treat inoperable liver tumors, allowing patients to live a more comfortable, longer life.
After completing her Master’s degree in biochemistry in 1989, Dr. Shukla chose to raise her two children and put her career on hold. A few years later, she picked up where she left and resumed her studies at the All India Institute of Medical Sciences to pursue a Ph.D. in Radiochemistry from the Department of Nuclear Medicine.
“Most of what I do,” Dr. Shukla said, “comes from a sense of duty. “As a child, I was brought up with values and morals inherited from my parents, and it was a result of those values and morals that I grew up wishing to make this world a better place to live.”
Inspired by her father, a university professor in the field of agriculture, she said she owes her problem-solving abilities to her mother.
“My love in the field of medicine initially inspired me that life is precious, regardless of being rich or poor. It was this feeling that first propelled me to pursue research in the field of cancer.”
Microsphere Cold Kit for Selective Intra-Arterial Radionuclide Therapy
Now 54, Dr. Shukla has been an Additional Professor at the Post Graduate Institute of Medical Education & Research, Chandigarh, since 2011. Her invention, the Microsphere cold kit for Selective Intra-arterial Radionuclide Therapy, was developed in 2013, in collaboration with other departments, including Hepatology, Radiodiagnosis, and Nuclear Medicine.
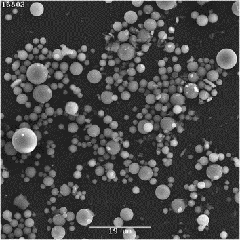
“The formulation is administered by delivering radioactive microspheres to tumors through an artery, resulting in effective tumor damage, while limiting the radiation injury to the unaffected parts of the liver,” she explained. The formulation also serves in palliative care. “Patients and their families believe that even one extra comfortable day with their loved ones is a godsend and with my formulation, I strive to make that extra time more congenial and allow them to live comfortably.”
Liver cancer often goes unnoticed for considerable periods of time, Dr. Shukla explained. There are no obvious symptoms during the onset of the disease, as such, it is diagnosed at a later stage, with extended collateral damage to the body.
The formulation is administered in a single sitting, reducing the procedural cost and lessening pain for patients, she further explained.
Making Liver Cancer Treatment Affordable thanks to Patent Registration
The formulation was patented to offer affordable treatment to patients, costing about 10 percent of the existing formulations, which were imported, with similar efficacy, she said. Affordability of available formulations is still out of reach of the common man, she added.
“IP has a major role in protecting our idea and products. It also helps us in the commercialization of products and makes [them] globally available for clinical use,” she explained.
To date, more than 50 patients have received the Selective Intra-arterial Radionuclide Therapy. The formulation is being used at the Post Graduate Institute of Medical Education & Research, but several other institutes have shown interest, she said. Dr. Shukla now hopes that her formulation will catch global attention.
Contrast Agent to Help Diagnose and Treat Cushing Disease
Alongside her research on radionuclide therapy, Dr. Shukla also worked with her endocrine colleague on a diagnostic imaging agent for Cushing’s disease, in which a pituitary tumor causes the body to make too much cortisol with numerous side effects.
The two imaging agents developed assist in the diagnosis and precision surgery of the disease. One imaging agent (68Ga-CRH) is already patented, and the second one is in the process. Both patents are filed nationally as well as internationally.
Source: WIPO

 Client Focus
Client Focus