Background
Back in 1874, a Norwegian pharmacist, Morten Nyegaard, founded Nyegaard & Co., a small pharmaceutical company initially working as an agent of products from foreign companies. Along the way,

(Picture: Madrid Express)
he introduced some 900 products from Norway to the rest of Scandinavia and created a pharmaceutical research center to produce new drugs. The privately owned company was later renamed Nycomed®. Over the years, Nycomed turned into a global pharmaceuticals company with strong presence in Europe, in the emerging markets of Russia and the Commonwealth of Independent States, Asia and Latin America.
Research and Development
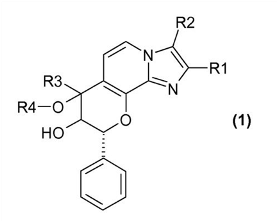
The company’s research and devleopment (R&D) strategy is driven by the search for business opportunities at all stages of product development and discovery. Its internal expertise is based on a flexible in-house R&D program that ranges from discovery, pre-clinical stages, phases of clinical research and registration with competent authorities. Its key products include drugs to combat osteoporosis and acid-related gastrointestinal diseases, drugs designed to stimulate wound healing, and an anticoagulant used in cardiology. Nycomed’s R&D programs operate out of three sites in Europe and one in India. New products emerge both from the company’s internal sources and from external partners.
Partnerships and Licensing
Unlike many of its competitors, Nycomed focuses less on creating new products and more on expanding the reach of its existing products, including those that it licenses in from other companies. The cornerstone of Nycomed’s business and growth strategy is based on long-term partnerships with other drug developers. The company aims to expand its drugs portfolio through in-licensing agreements or through joint development with external partners, and expects most of its future products to originate from outside Nycomed. Other companies are interested in collaborating with Nycomed because of its financial strength, global presence and strong research and development skills.
Most of Nycomed’s in-licensing partners are pharmaceutical and small biotech companies from the United States that would otherwise find it difficult to enter into complicated European markets. Nycomed believes that this strategy is mutually beneficial for the company itself and for its external partners; foreign companies benefit from the opportunity of bringing their novel products to new markets while Nycomed benefits from the exciting discoveries made by small biotech firms.
Nycomed also relies on mergers and acquisitions whenever such schemes offer business opportunities. For example, in 2007 Nycomed purchased German-based Atlanta Pharma AG. Following the merger, the combined group has continued to operate under the Nycomed corporate name and established its headquarters in Zurich, Switzerland. The same year it also signed an agreement to acquire Bradley Pharmaceuticals, Inc. of the United States. The acquisition facilitates Nycomed’s promotion of its business interests in the United States. In 2009, Nycomed purchased 20 branded generic products in several Central and Eastern European countries from Sanofi-Aventis and Zentiva, which significantly strengthened Nycomed’s market position, particularly in the Czech Republic and Slovakia.
On the out-licensing side of Nycomed’s business strategy, the company offers a variety of sophisticated technologies and services for external partners to develop, manufacture and commercialize. A number of Nycomed products have been licensed out to several companies including Forest Laboratories, Merck & Co., and Sepracor. The licensing-out dealings have benefitted Nycomed not only in terms of upfront payment and subsequent royalties, but also in expanding the market for its own products.
Commercialization
Nycomed favors products that can be marketed in multiple countries and with a sales potential of over Euro (€) 150 million (US$ 192 million). Rather than trying to compete across the board with the big pharmaceutical companies, it has chosen to compete in particular markets and with carefully selected products. This strategy has proven to be efficient. The company’s best-selling product, CalciChew, (a combination of calcium and vitamin D3), is the top calcium product in Europe with a 40 percent market share. Pantoloc/Zurcale (for gastro-intestinal diseases), which it licenses from another company, is the number one seller in its class in Austria and number two in the Netherlands and Belgium.
Patents
Nycomed has a clearly articulated patent strategy, which is formally approved by the office of the chief executive officer. The patent policy serves as a mission statement and guides the company’s decision-making.
The company has a set procedure on patenting new products. Patent applications are first filed with national IP offices, where costs are low and the procedures familiar. This gives the company a year to decide whether to expand the reach of the intended patent protection. If so, Nycomed then typically files patent applications in Canada, China, all European countries, Japan, Russia and the Commonwealth of Independent States, the United States and other selected countries. The company is a frequent user of the services provided under the Patent Cooperation Treaty (PCT) system, and as of 2010 it has filed more than 400 PCT applications.
When deciding not to file a patent application for a particular process, Nycomed either tries to keep it secret (while recognizing the difficulty in effectively preventing employees who join competitors from divulging such secrets) or publishes information about the process to put it in the public domain and thus keep it from being patented by others.
Trademarks
Nycomed puts special emphasis on trademark protection of its products. All the names of the company’s products are registered trademarks and are protected either internationally or in individual countries. For example, CalciChew®, one of most popular medicines of the company, is internationally protected. The logo of the company is also internationally protected through registration with the Madrid system.
IP Management
Marketing products successfully also means protecting the company’s associated intellectual property rights (IPRs). In 2003, Nycomed estimated that 46 percent of its turnover came from patent-protected products. In 2006 that figure rose to between 55 and 60 percent and is expected to continue to rise as additional new products are patented.
Managing intellectual property (IP) is complicated and costly, with a portfolio consisting of some 500 patents plus 800 or so registered trademarks, which provide some protection from generic competitors once patents expire. The company budgets € 12.3 million (US$ 15.7 million) per year to file, protect and exploit patents. Litigation is especially expensive. A single action in a single country costs roughly € 1.5 million (almost US$ 2 million), and much more for litigation covering several countries and appeals. But Nycomed considers the money well spent and has yet to lose a lawsuit.
Each year the company conducts a major IP review. Any patents in its portfolio that are no longer generating revenue are checked for licensing potential. If there is none, they may be abandoned by the company or assigned to a university. Competitors’ patents are also scrutinized to ensure they are not infringing on the company’s patents and vice versa. The annual IP review can also identify new technologies and ideas to pursue.
IP Infringement/ Enforcement
Protonix®, one of Nycomed’s globally-popular products for reducing or preventing the production of gastric acid, is owned by Nycomed (United States Patent No. 4,758,579) and licensed to Wyeth (an American pharmaceuticals company, now part of Pfizer Inc.). In 2007, Protonix® sales reached US$ 1.9 billion. Sales, however, decreased considerably when two other pharmaceuticals companies, Teva Pharmaceuticals Ltd. and Sun Pharmaceuticals Ltd., started selling a generic product which infringed on Nycomed’s patent in late 2007 and early 2008 respectively. Nycomed and Wyeth filed a lawsuit against the infringing acts of Teva and Sun.

Patent-protected products, such as this wound-healing and pain treatments, generate some 60 percent of Nycomed’s annual turnover.
In July 2010, the United States District Court upheld the lower court jury verdict of April 2010 in favor of Nycomed and Pfizer, confirming that Nycomed’s Protonix® patent is valid. The verdict rejected the defendants’ allegations that the patent was invalid due to its “obviousness” and double-patenting. Upon confirmation of the validity of the patent by the District Court, Nycomed will pursue damage claims against Teva and Sun.
Business Results
Starting as a very small private business, Nycomed has gone through a long journey to establish itself as a leading pharmaceutical company, ranking 28th globally. Its pragmatic business strategy has facilitated its remarkable sales achievement: in 2009, total sales reached € 3.2 billion, with an adjusted EBITDA (earnings before interest, taxes, depreciation, and amortization) of € 1.1 billion. Nycomed is the 16th largest provider of over-the-counter medicines in the world.
The company has 12,000 employees and its products are sold in more than 100 countries. Its affiliates are located in more than 50 countries. Nycomed’s manufacturing takes place in 16 sites. Global products are manufactured in 5 centers of excellence in Europe. There are 11 production sites for regional products in fast-growing markets such as Brazil and Mexico. Russia is currently the biggest market of Nycomed, and consequently the company is in the process of establishing a new high-profile production plant in Russia.
Well-Formulated IP Strategy Leading Toward Business Success
Nycomed’s experience shows how a well thought-out and executed IP strategy can keep a company competitive in a market dominated by much larger rivals. The company realizes that IP assets are equally valuable as financial assets, and accordingly Nycomed’s patent strategy is very much linked to its business strategy.
Source: WIPO

 Client Focus
Client Focus